COLLIE HEALTH
Like people, dogs can be subject to a number of diseases. Here is a brief overview of a few pertinent to the Collie breed.
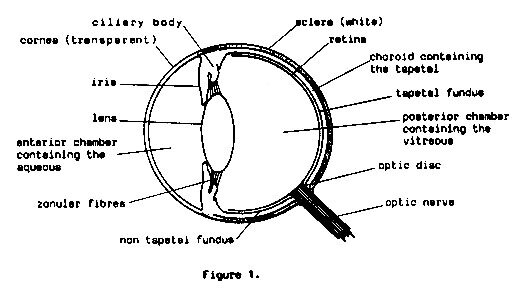
collie eye anomaly
Collie Eye Anomaly (CEA) is the common-term applied to a set of abnormalities located near the fundus at the back of the eye. All Collie puppies should be tested by a certified canine ophthalmologist (regular vets do not have the specialist requirements to diagnose) for CEA between 6-8 weeks of age.
Whilst the disease is complex, it can be classified into three main categories; Choroidal Hypoplasia (CH), Coloboma, and Retinal Detachment.
The most minor of these categories is Choroidal Hypoplasia (Chorioretinal change). Upon clinical examination, the ophthalmologist may notice an area of diminished pigment on the fundus which is observed as a pale area, with fewer blood vessels present. The size of the pale area (which is lateral to the optic disc) will determine the severity of the score. A reading of ‘Mild Choroidal Hypoplasia” appears to be the most common expression of the CEA complex. This abnormality in the choroid regardless of size does not interfere with vision. Choroidal Hypoplasia is non-progressive meaning it will not get worse, and dogs diagnosed with a mild degree of CEA will often read as normal in subsequent examinations as the area fills with pigment, with no vision deficits.
The second category is most commonly termed Coloboma. A coloboma (which may be either unilateral or bilateral) appears as a hole or indentation in the region of the optic nerve, where the choroid, tapetum & or retina may be abnormally formed upon examination. The size of the coloboma/pitting will determine the degree to which eyesight is affected, and whilst they are not necessarily progressive, the presence of larger colobomas can lead to retinal detachment. It was previously understood that colobomas were found in dogs with chorioretinal change, however there have been cases of Collies found to have colobomas; who had no coreoretinal change (CH) detected.
Retinal Detachment represents the most severe form of CEA and can be present in either or both eyes. Dogs with retinal detachment will be blind in that eye, as the retina is not properly attached and can therefore not perform the sensory task of sight.
It is thought that CEA, which affects many herding breeds, has been present for a very long time and for a breed as historically steeped as the Collie, the problem became unknowingly entrenched amongst the Collie population until the advent of modern technology made assessing breeding stock commonplace. Breeders have worked tirelessly to decrease the instances of CEA by eliminating the most severely affected dogs from their breeding programs; whilst still juggling all other considerations and the need to protect genetic diversity.
To this end, all puppies and breeding stock should be eye tested by a board certified canine ophthalmologist to reduce the incidence of vision affected Collies.
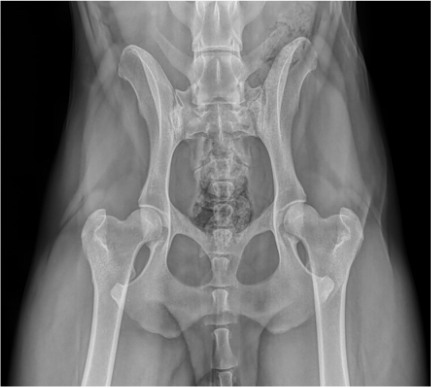
HIP DYSPLASIA
Hip Dysplasia is the abnormal growth and development of the hip joint. It is a polygenetic condition considered genetic, but affected by environmental influences such as excess weight, diet, exposure to slippery surfaces, injury and improper exercise which interact and impact the growth of the puppies hip joint. Consequently, this leads to rapid wear and tear of the hip joint and eventually arthritic change.
The Australian Veterinary Association (AVA) has a scoring scheme that is Australia wide with averages produced for each breed. Scoring is done on 9 different points of the hip joint anatomy and each area (apart from the caudal acetabular edge which is out of 5) is scored out of a possible 6 points. The lower the score, the better the hips. The minimum score per hip is 0 and the maximum score per hip is 53, with an overall total of 106.
The genes that are responsible for HD remain a mystery. Sires and Dams with perfect hips can produce a dysplastic puppy, the same way dysplastic parents can produce a puppy with perfect hips; indicating that environmental factors are responsible for expressing the disease. It's unlikely that researchers are going to discover an easy genetic solution to the problem of hip dysplasia. It is a complex trait that is influenced by both genes and environment as mentioned, and there is no simple solution just over the horizon. With that in mind, genetic progress can be made by using selection strategies that are as efficient and effective as possible such as ‘estimated breeding values’ (EBVs). One great advantage of using EBVs is that the genes responsible for a trait don't need to be known; you need only a pedigree database and information about affected animals. Breeding dogs with good hip scores, from families of dogs with a consistent history of good hip scores, is adopted as a practical approach.
Elbows can also be scored - and are done generally at the same time the hips are being x-rayed.
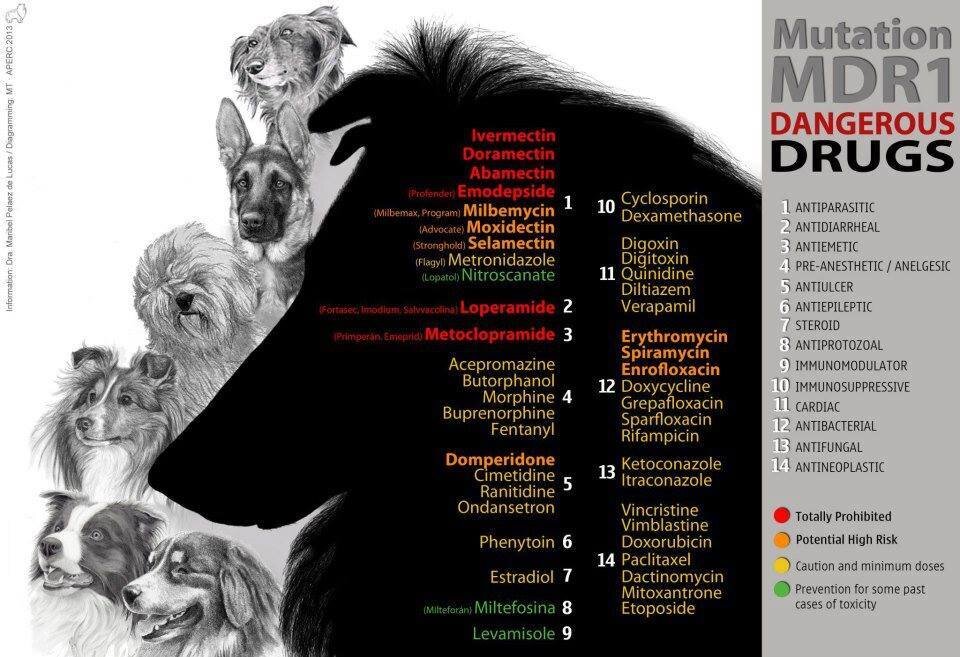
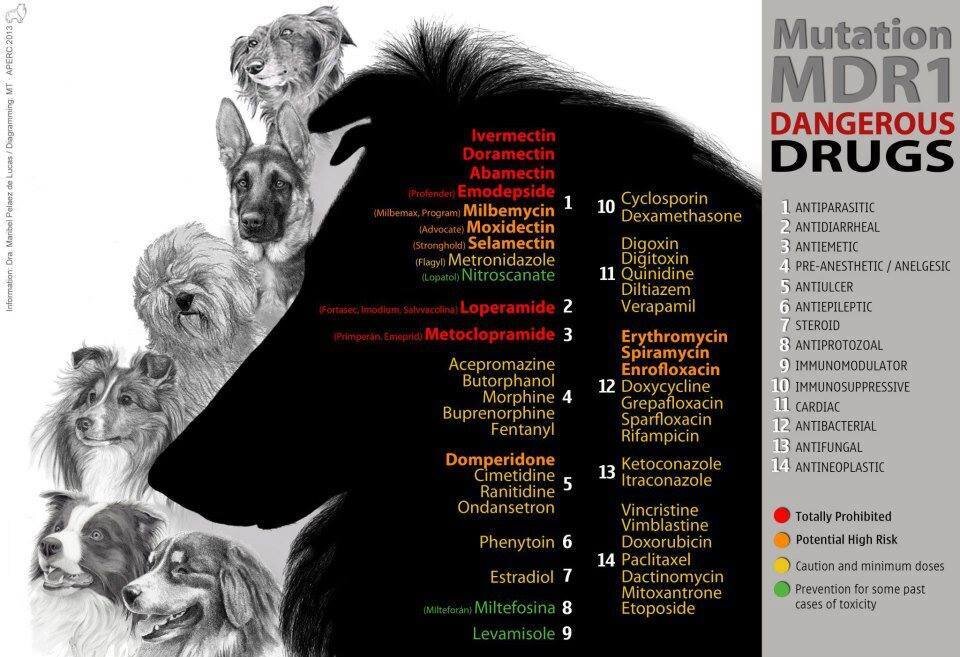
multi drug resistance mutation (mdr1)
Collies (and many herding breeds) can have a mutation in the MDR1 gene that makes them more sensitive to the negative effects of certain medications. These drugs include several antiparasitic agents (when given at high doses), the antidiarrheal agent loperamide (Imodium®), and several anticancer drugs. Dogs with MDR1 mutations will show negative effects such as increased neurologic effects from these drugs at doses that are readily tolerated by dogs without the mutation.
Ivermectin and other related drugs (milbemycin, selamectin) are commonly used in the prevention and treatment of parasites. They are common components of most canine heartworm preventives.
Sedatives, such as those commonly given as components of a balanced anesthetic protocol (acepromazine, butorphanol), may also show stronger effects in dogs with MDR1 mutation. This is a very subtle difference in degree of sedation and duration of sedation. These dogs can still receive typical anesthetic drugs safely; however, veterinarians may choose to use lower doses in dogs who are suspected or known to possess an MDR1 mutation. Chemotherapy drugs may also trigger more severe side effects in dogs with MDR1 mutation. These effects can include gastrointestinal toxicity and bone marrow suppression, even at low doses.
MDR1 is inherited in an autosomal incomplete dominant manner in dogs meaning that dogs only need to inherit one copy of the mutated gene to be at an increased risk of developing adverse reactions to certain medications. A simple DNA test exists, nevertheless, the simple recommendation is - no matter what the MDR1 status, treat as if they were affected.
degenerative myelopathy
Degenerative Myelopathy (DM) is a progressive neurological disorder that affects the spinal cord of dogs. The disease often begins with an unsteady gait, and the dog may wobble when they attempt to walk. As the disease progresses, the dog's hind legs will weaken and eventually the dog will lose the use of it’s rear legs. Degenerative Myelopathy moves up the body, so if the disease is allowed to progress, the dog will eventually be unable to hold his bladder and will lose normal function in the front legs. Fortunately, surprisingly there is no direct pain associated with Degenerative Myelopathy. This is a geriatric disease and the onset of Degenerative Myelopathy generally occurs at an average age of about 10-12 years.
Degenerative myelopathy is considered a complexly (polygenic) inherited disease. Researchers at the University of Missouri have developed a DNA test; looking for mutation in the SOD1 gene that identifies the risk factor in the development of DM. Two copies of this autosomal recessive susceptibility gene must be present to put the dog at an increased risk for the disease to express, however, it is to be noted that all individuals who are homozygous (have two copies) for this gene might not become affected. They are considered "at risk", but not necessarily genetically affected. This is known as ‘incomplete penetrance’ - meaning even dogs with two copies of the genotype may never be physically afflicted. That is because there are additional (as yet unidentified) genes that must also be present to produce clinical signs of DM, although onset is speculated to be immune mediated. At present time the only way to definitively confirm a diagnosis of DM is via necropsy.
On this topic, I have been in direct communication with Dr Jerold S Bell DVM (Chairman of the Hereditary Disease Committee - World Small Animal Vet Assn, Internationally renown Genetic consultant, Director - OFA, and adjunct professor of Genetics - Department of Clinical Sciences, Cummings School of Veterinary Medicine). Dr Bell was engaged by the Collie Health Foundation on a 4 year tenure to study Collie health and pertinent diseases. He advises the below -
“The high gene frequency of the SOD1 variant across breeds and the infrequent clinical presentation of DM in any breed causes much confusion in genetic counseling. With public acceptance of direct-to-consumer multiplex panel genetic testing for dogs, all panel tests include SOD1 variant test results regardless of their relevance to individual breeds and dogs. In all breeds (and mixed-breed dogs) a DM “at risk” result places a significant and unnecessary emotional burden on owners who believe that their family member will develop DM and die from the disease – which is highly unlikely to occur. A decision to euthanize a dog due to a DM “at risk” test result when differentials include common treatable diseases such as disc disease and musculoskeletal disease are unacceptable and borders on malpractice. In my breed health seminar to the Collie Club of America at their National I stated, "While there are documented sporadic cases of degenerative myelopathy in the Collie breed, DM does NOT exist as a breed-associated disease. The frequency of the sod1 mutation far exceeds the frequency of Collies that develop DM – thus making it a POOR PREDICTOR of disease liability."
PROGRESSIVE RETINAL ATROPHY (PRA-RCD2)
Progressive Retinal Atrophy (Rod-cone dysplasia 2) is an early-onset, inherited eye disease. PRA occurs as a result of degeneration of both rod and cone type photoreceptor cells of the retina, which are important for vision in dim and bright light, respectively. Affected dogs have abnormal thinning and degeneration of the retina beginning around 16 days of age. The rod type cells are affected first and affected dogs will initially have vision deficits in dim light (night blindness) and loss of peripheral vision noticeable at about 6 weeks of age. Other signs of progressive retinal atrophy involve changes in reflectivity and appearance of the tapetum (located behind the retina) that can be observed at 3-4 months of age on a clinical ophthalmology exam. As the disease progresses, cone photoreceptor cells also degenerate resulting in complete blindness by 6 to 12 months of age. The test for PRA-rcd2 is offered on various full DNA panels for the Collie Rough however I don’t believe the disease is apparent in Australia meaning a prevalence may have existed in bygone decades and has been bred out, or is potentially more apparent in overseas countries where it has perhaps not yet been eliminated.
Happy beach Collies, living their best life!
HEALTH & EDUCATIONAL SEMINARS
Collie Health Foundation and Collie Club of America 2022 Seminar with Dr. Jerold Bell Part 1
Collie Health Foundation and Collie Club of America 2022 Seminar with Dr. Jerold Bell Part 2